Pancreatic Cancer Diagnostic Market 2030: Exploring Global Market Dynamics
The global pancreatic cancer diagnostic market size is expected to reach USD 3.90 billion by 2030, according to a new report by Grand View Research, Inc. Furthermore, the industry is expected to expand at a CAGR of 6.53% over the forecast period.
The global pancreatic cancer diagnostic market size is expected to reach USD 3.90 billion by 2030, according to a new report by Grand View Research, Inc. Furthermore, the industry is expected to expand at a CAGR of 6.53% over the forecast period. The introduction of innovative products, coupled with the rising need for early disease diagnosis and increasing research activities to develop advanced diagnostic solutions, is anticipated to drive the global market.
For instance, in August 2021, Immunovia, Inc. received approval from the Massachusetts Department of Public Health to start testing patients for pancreatic cancer, using the IMMray PanCan-d test. It is a laboratory-developed test for the early detection of pancreatic cancer. The market is projected to witness lucrative growth opportunities owing to increased research funding, technological advancements in diagnostic tests, and rising efforts from manufacturers to develop precise testing solutions. For instance, in December 2022, Bluestar Genomics, Inc. announced the initiation of one of the largest clinical trials for the early detection of pancreatic cancer.
In addition, the growing demand for developing novel automated and accurate diagnostic solutions to reduce the disease burden worldwide has gained momentum in recent years. Research related to novel biomarker identification, nanoparticle utilization for pancreatic cancer, and the introduction of artificial intelligence (AI) in diagnosis processes are factors projected to fuel the growth. In addition, the incorporation of AI in imaging pathology and biomarkers tests will boost the adoption of diagnostic tests for detecting pancreatic cancer.
Get a preview of the latest developments in the Pancreatic Cancer Diagnostic Market; Download your FREE sample PDF copy today and explore key data and trends
For instance, in April 2022, Fujitsu collaborated with Southern Tohoku General Hospital in Fukushima, Japan to develop AI solutions that detect pancreatic malignancy from CT scans at an early stage. Rising investments by key market players in the production of effective and advanced diagnostic tools and various strategic initiatives undertaken by them are likely to create new avenues for market expansion. For instance, in March 2023, Prestige Biopharma Limited developed a first-in-class diagnostic kit for detecting pancreatic cancer. The test helps diagnose the disease at an early stage to improve treatment outcomes and survival rates globally.
Similarly, a favorable regulatory framework for diagnostic tools is another factor supporting market expansion. For instance, in January 2023, the U.S. FDA granted a breakthrough device designation to the OverC Multi-Cancer Detection Blood Test (MCDBT) for the early diagnosis of various forms of cancers, including lung, liver, esophageal, and pancreatic cancer in adults with average and intermediate risk.
Pancreatic Cancer Diagnostic Market Report Highlights
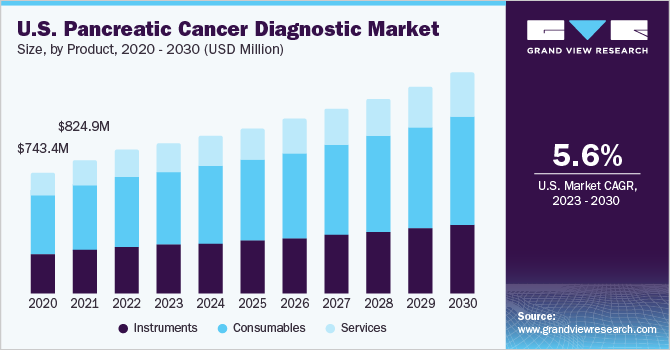
- The consumables segment held the largest revenue share in 2022, owing to the rising adoption of consumables in diagnostic procedures, increased R&D investments, and growing instances of launches of technologically advanced diagnostic kits and reagents
- The imaging test segment accounted for the largest market revenue share in 2022 due to its ability to allow healthcare professionals to detect and diagnose pancreatic cancer at earlier stages and the high preference for imaging modules to position tumors
- Based on end-use, the hospital segment dominated the market in 2022, whereas the laboratories segment is projected to be the fastest-growing segment over the forecast period
- Asia Pacific is projected to exhibit the fastest growth over the forecast period due to the high unmet needs of the patient population and the increasing investment of key market players in the region
Pancreatic Cancer Diagnostic Market Segmentation
Grand View Research has segmented the global pancreatic cancer diagnostic market based on product, test type, cancer type, end-use, and region:
Pancreatic Cancer Diagnostic Product Outlook (Revenue, USD Billion, 2018 - 2030)
- Instruments
- Consumables
- Services
Pancreatic Cancer Diagnostic Test Type Outlook (Revenue, USD Billion, 2018 - 2030)
- Imaging Test
- Biopsy
- Blood Test
- Others
Pancreatic Cancer Diagnostic Cancer Type Outlook (Revenue, USD Billion, 2018 - 2030)
- Exocrine
- Endocrine
Pancreatic Cancer Diagnostic End-use Outlook (Revenue, USD Billion, 2018 - 2030)
- Hospitals
- Clinics
- Laboratories
- Others
Pancreatic Cancer Diagnostic Regional Outlook (Revenue, USD Billion, 2018 - 2030)
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Asia Pacific
- Japan
- China
- India
- South Korea
- Australia
- Thailand
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
Curious about the Pancreatic Cancer Diagnostic Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.