Mastering Global Medical Device Compliance: How I3CGLOBAL Simplifies ISO 13485
In the rapidly evolving medical device industry, staying compliant with global regulatory standards
In the rapidly evolving medical device industry, staying compliant with global regulatory standards is not just an advantage—it is a necessity. Manufacturers must navigate complex frameworks, from quality management requirements to market-specific approvals, before making their products available worldwide. This is where I3CGLOBAL emerges as a trusted partner, guiding manufacturers through every step of the compliance journey. Their expertise in ISO 13485 Certification, FDA QMSR, EU Representative services, and FDA 510k submissions ensures seamless, efficient, and reliable regulatory success.
Why ISO 13485 Certification Matters for Medical Device Companies
Achieving ISO 13485 Certification is fundamental for any medical device manufacturer aiming to demonstrate competence in producing safe, high-quality devices. This globally recognized standard focuses on strict quality management system (QMS) requirements that control every aspect of the product lifecycle—from design and development to manufacturing, storage, and distribution.
The significance of ISO 13485 Certification extends beyond compliance. It enhances customer trust, boosts international market opportunities, and reduces risks associated with product recalls or regulatory non-conformities. I3CGLOBAL supports companies in developing, implementing, and maintaining a robust QMS aligned with ISO guidelines. Their expert consultants ensure documentation accuracy, gap assessments, internal audits, and readiness for certification audits.
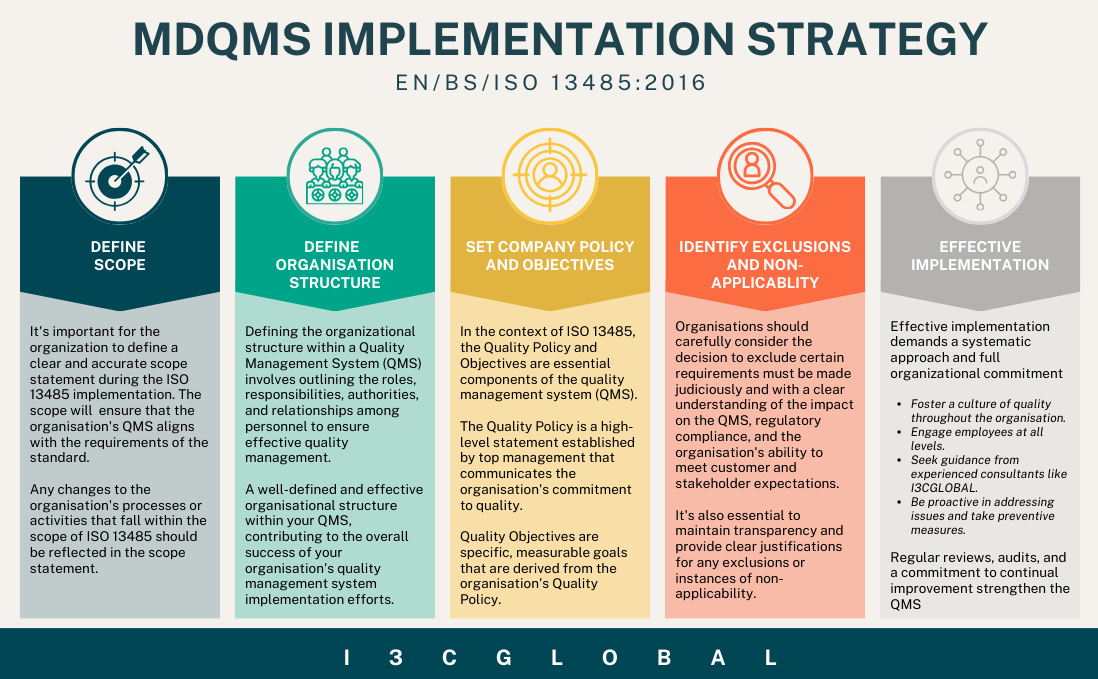
With their technical expertise and hands-on experience, I3CGLOBAL helps organizations navigate the complexities of regulatory pathways efficiently. Their tailored approach ensures that companies of all sizes—from startups to large enterprises—are fully prepared for certification with minimal disruption to operations.

Understanding FDA QMSR: A Crucial Standard for U.S. Market Entry
For manufacturers planning to enter the U.S. medical device market, compliance with the FDA QMSR (Quality Management System Regulation) is mandatory. Recently harmonized with ISO 13485, the new FDA QMSR integrates key elements of the international standard while maintaining FDA-specific requirements such as complaint handling, labeling controls, and medical device reporting.
Navigating these requirements can be challenging, especially for companies unfamiliar with U.S. regulations. I3CGLOBAL bridges this gap by offering comprehensive consulting services that help manufacturers understand and implement the QMSR framework effectively. Their team conducts gap analyses, provides compliance strategies, and supports documentation preparation to ensure smooth FDA inspections.
The alignment of FDA QMSR with ISO 13485 presents an opportunity for manufacturers to streamline compliance efforts—and I3CGLOBAL ensures that you maximize this advantage.
EU Representative: A Mandatory Requirement for MDR Compliance
Medical device manufacturers located outside the European Union must appoint an EU Representative to sell their products in EU markets. This representative acts as a liaison between the company and regulatory authorities, taking responsibility for ensuring MDR (Medical Device Regulation) compliance, technical file maintenance, incident reporting, and regulatory communication.
Appointing the right EU Representative is essential because their competence directly impacts your product’s market acceptance and regulatory standing. I3CGLOBAL offers professional EU Representative services backed by deep regulatory knowledge and years of experience handling compliance for global clients.
With the ongoing challenges posed by EU MDR, including tighter clinical evaluation and post-market surveillance requirements, partnering with I3CGLOBAL ensures uninterrupted market access and full regulatory alignment.
FDA 510k Submission: Opening Doors to the U.S. Market
Most Class II medical devices require a FDA 510k submission before they can be marketed in the United States. This process involves demonstrating that a new device is substantially equivalent to a legally marketed predicate device. Preparing a FDA 510k submission demands precision, technical expertise, and thorough understanding of FDA expectations.
I3CGLOBAL specializes in guiding manufacturers through every stage of the FDA 510k process—including predicate identification, risk analysis, performance testing, labeling reviews, and submission preparation. Their team ensures the application is complete, accurate, and aligned with FDA guidelines, significantly improving approval timelines. This reduces the likelihood of refusal-to-accept (RTA) or deficiencies, helping manufacturers bring their devices to market faster.
Their experience across a broad range of medical devices—from diagnostic equipment to implants—ensures that your submission is handled by experts who understand the intricacies of FDA regulatory frameworks.
Why Choose I3CGLOBAL as Your Compliance Partner
In a world where medical device regulations are growing increasingly complex, having an expert by your side is crucial. I3CGLOBAL stands out for its:
1. Technical Expertise Across Global Markets
Their team understands the nuances of ISO 13485 Certification, FDA QMSR, EU Representative responsibilities, and FDA 510k submissions.
2. End-to-End Support
From documentation to audits, testing coordination, and regulatory communication, I3CGLOBAL covers every aspect of compliance.
3. Proven Track Record
With years of helping clients achieve successful approvals and certifications, they bring reliability and trust to the regulatory process.
4. Customized Solutions
Every medical device is different. I3CGLOBAL designs tailored compliance strategies to match your product’s unique requirements.
In the medical device industry, regulatory compliance is the foundation of global success. Whether you are expanding into the EU, entering the U.S. market, or strengthening your quality management system, I3CGLOBAL offers the expertise and support you need. With their guidance across ISO 13485 Certification, FDA QMSR, EU Representative services, and FDA 510k submission pathways, manufacturers can confidently navigate the regulatory landscape and achieve long-term growth.