Clinical Trials Support Services Market Analysis by Sponsor and Regions
The clinical trials support services market is on a strong upward trajectory, propelled by rising R&D investments, increased outsourcing to CROs, and advancements in clinical research technologies.
The global clinical trials support services market was valued at USD 23,881.4 million in 2024 and is expected to reach USD 37,161.2 million by 2030, registering a CAGR of 7.7% from 2025 to 2030. Growth is driven by increasing demand for clinical trials in emerging economies, rising R&D investments, and the expanding presence of contract research organizations (CROs).
Pharmaceutical companies continue to escalate their R&D expenditures, largely due to ongoing patent expirations. While a standard patent lasts 20 years, pharmaceutical patents typically allow generic entry after 10 years, prompting companies to accelerate drug development pipelines. This increasing focus on innovation is contributing significantly to market expansion.
Clinical trials support services play a critical role in drug development, offering solutions such as assay design, clinical testing, site optimization, investigational drug storage, dosage calculation, and kit management. These services also include preclinical preparation, site assistance, drug blinding, patient recruitment, coordination activities, and returned-medication reconciliation.
Key Market Trends & Insights
- North America led the global market in 2024 with a 48.80% share.
- The U.S. represented the largest share within the North American market.
- Phase III trials accounted for the largest share by phase, representing 54.43% of total revenue.
- By service type, clinical trial site management dominated with a 45.73% share in 2024.
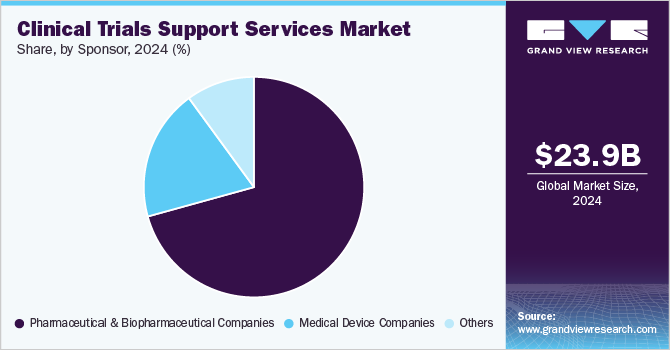
- By sponsor, pharmaceutical & biopharmaceutical companies led the market with 70.80% of total revenue.
Download a free sample PDF of the Clinical Trials Support Services Market Intelligence Study by Grand View Research.
Market Size & Forecast
- 2024 Market Size: USD 23,881.4 Million
- 2030 Market Size Projection: USD 37,161.2 Million
- CAGR (2025–2030): 7.7%
- Leading Region (2024): North America
Competitive Landscape
Leading market players are engaging in expansions, acquisitions, partnerships, and technology-driven initiatives to reinforce their market position.
- January 2024: IQVIA launched One Home for Sites, a unified technology platform providing clinical research sites with a centralized dashboard to manage all trial activities.
Prominent Companies
- Charles River Laboratories International, Inc.
- Wuxi Apptec, Inc.
- IQVIA Holdings, Inc.
- Syneos Health, Inc.
- Eurofins Scientific
- PPD, Inc. (Pharmaceutical Product Development)
- ICON plc
- Laboratory Corporation of America Holdings (Labcorp)
- Alcura
- Parexel International Corporation
Recent Developments
- July 2024: DocMode Health Technologies launched new clinical research services aimed at offering end-to-end solutions including trial management, regulatory compliance, and data analytics. These services are positioned to help pharmaceutical companies, healthcare institutions, and researchers streamline and enhance clinical study execution.
Explore Horizon Databook – the world’s most comprehensive market intelligence platform by Grand View Research.